shelf life calculator for pharmaceutical products
Each batch is distinguished by its own shelf life which can be called the true batch shelf life. Expiration Dates - Questions and Answers.

Use Of A Two Tiered Degree Hour Measure For The Management Of Investigational Medicinal Product Over Temperature Excursions Using Mean Kinetic Temperature Mkt Ivt Gmp Stability Testing
Thus the true shelf-life denoted byθ is the solution ofηαβx hence.

. The date given on the product packaging which represents the end of the shelf life Stability of pharmaceutical products. At least three distinct steps are necessary to gain knowledge and understanding of a product or process. A period of time during which if stored correctly product is expected to retain acceptable chemical physical and microbiological stability SPC.
Establishing the Shelf Life of Pharmaceutical Products. θη αβNotethatαis the average drug characteristic at the time of manufacture iex 0 which is usually larger thanηThusθ0. Up to 10 cash back Batch and Product Shelf Life.
Accelerated aging studies can be used for shelf life determination but must later. C Co - k x t C518 - 0267 x t v. Fresh Cooking Conversion Calculator in 2020 Baking.
Calculator Accelerated Aging Time AAT Accelerated Aging oftentimes referred to as Accelerated Shelf-Life Testing is commonly used in the medical device industry to accelerate the effects of time on a Sterile Barrier System to establish Shelf Life parameters. Let ˆθbe an estimator of the true shelf-lifeθbased on y. 2 years 36 months Expiration date expiry date.
Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. The shelf life of a product may vary between different countriesregions depending on regulatory requirements.
What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. Why are expiration dates important for consumers to pay attention to. A pharmaceutical product is typically manufactured in batches.
Shelf life calculator for pharmaceutical products. QUANTILE REGRESSION CALIBRATION. The batch is a single sample of the pharmaceutical products manufacturing process at.
New Drug Substances and Products presented at the FDA - GPhA Spring workshop in 2012 2013. Shelf life is determined by the evaluation of whole stability data of the product. The quality within each step influences the quality of the resulting knowledge.
Pacific coast composites shelf life calculator is provided in order to help our customers determine the remaining shelf life of their product. ICH Q1E guideline provides guidance for the estimation of the shelf life of the pharmaceutical products and substances. The table below lists the current regulatory reporting categories for shelf-life extensions of pharmaceutical products in different countries.
This can also be used to determine the shelf life of a product prior to purchase. Accelerated stability testing and shelf-life calculation. Enter all three dates to the left to see the remaining shelf life.
Decision Tree for retest period or shelf life estimation excludes frozen DsDp Use of tables. This can also be used to determine the shelf life of a product prior to. SHELF LIFE CALCULATION b N x ƩXY -ƩXx Ʃy -3024 N x ƩX² - ƩX² 11340 b -0267 mgmonth k 0267 a ӯ-b x x a 518 ivThe equationfora straightline bestfitis therefore.
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data. Minimum data of three batches are used to estimate the shelf life of pharmaceutical products. Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months.
The variance estimateS²xyrepresentsthe variabilityof tabletpotencyatafixedtimeassumingitisequivalentacross all time points. Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions. A batch is a fixed quantity of product for example 100000 tablets.
Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product. Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by. Data may request an extension of the product shelf life later as a post-approval change and submit the required supporting information.
Drug expiration dates reflect the time period during which the product is known. The International Conference on Harmonisation ICH of Technical Requirements for the Registration of Pharmaceuticals for Human Use guidance document Q1AR2 ICH Q1A defines shelf life as The time period during which a drug product is expected to remain within the approved shelf life specification provided that it is stored under the conditions defined on. Expiry with date in days in months or in years.
As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various.

How To Calculate Expiration Dates In Excel
Change To Read 1160 Pharmaceutical Calculations In Pharmacy Practice 1s Usp38 Change To Read Table Of Contents Introduction Calculating Amounts Of Active Ingredients Dosage Calculations Use Of

Shelf Life Study Of Extemporaneously Prepared Omeprazole Oral Suspension Makeen Ha Pancholi Ss Ali Ms Alam Mi Alam Ms Redaie Am Albarraq Aa Al Bratty M Kadomi Yj Qasim Attiya Ma Aribi Sh

Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram
Accelerated Aging Calculator Shelf Life Calculator Packaging Compliance Labs

How To Calculate Expiration Dates In Excel

How To Calculate Expiration Dates In Excel

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram
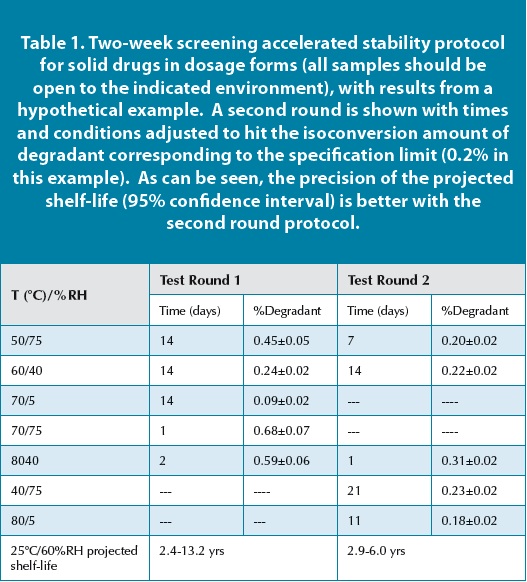
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

How To Calculate Expiration Dates In Excel

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

How To Calculate Expiration Dates In Excel
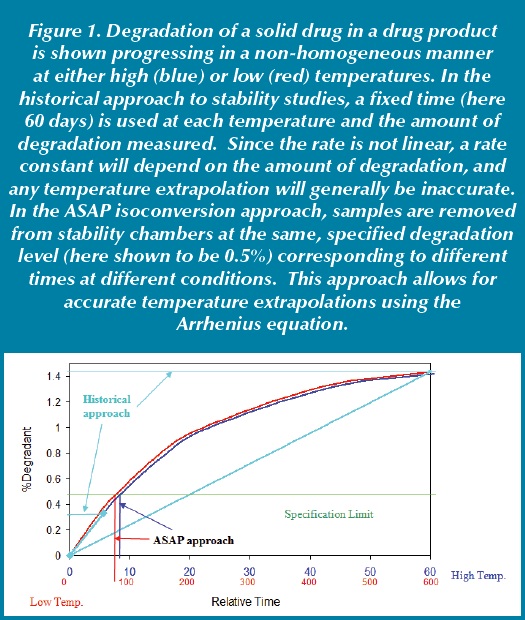
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram